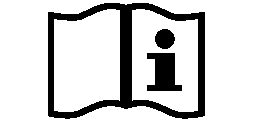
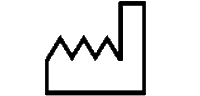
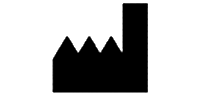
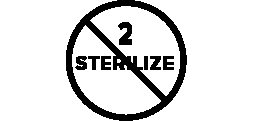
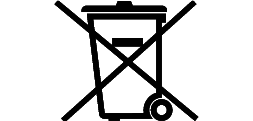
Symbols Glossary
Compliance to global labeling regulations is a paramount priority. Our labeling follows global regulations including the Federal Drug Administration’s (FDA) General Device Labeling 21 CFR Part 801 and EU Medical Device Regulations (MDR) Article 23.1, Chapter III of Annexure I.
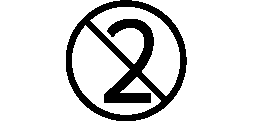
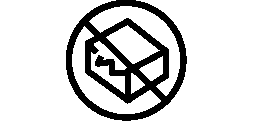
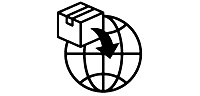
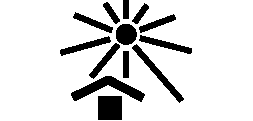
We use the following graphics, as required, to label our Medical Devices. These symbols are part of the ISO 15223-1:2021, EN 15986:2011, WEEE Directive 2012/19/EC, IEC 60601-1:2012 or MDR 2017/745 denoting the symbols and information to be provided on the labels of medical devices.