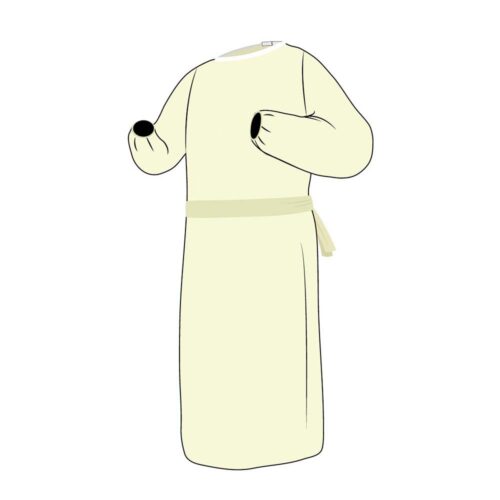
Invenio’s lightweight ChemoGuard® chemotherapy gown maximizes protection from hazardous drugs.
ChemoGuard® Chemotherapy Gown
Description
Invenio’s lightweight ChemoGuard® chemotherapy gown is designed to maximize protection while compounding, administering, managing waste, or cleanup of chemotherapeutic drug spills. With distinguished branding to identify specific useability with chemotherapy drugs our chemotherapy gown prioritizes comfort and safety featuring a full coverage closed back, a generous cut, knit cuffs, and a tie neck closure.
Additional information
| Dimensions | N/A |
|---|